By Jennifer Hermitage, Market and Application Chemist
What can we learn from other industries, and what does the future hold?
Over the past decade, as consumers have become more aware of the dangers of sun-exposure, sun protection products have become a mainstay of daily skincare rituals. Thankfully, the days where sunscreen was only worn while on holiday are gone and the benefits of wearing high sun protection factor (SPF) products daily have gone mainstream. There is now a substantial market for products containing SPF including moisturisers, lipsticks, make-up, shampoos and conditioners. It has been reported that the market for sun protection generated revenues of US $11.52 billion in 2024 and is projected to experience a compound annual growth rate (CAGR) of 4.14% between 2024 and 2028.1

However, while the ever-increasing breadth of products available is certainly good news for the prevention of skin cancers, every formulator knows that behind these new products are months of research and development to ensure both product stability and suitability, as well as regulatory compliance. However, recent bad press2 around sunscreen performance and SPF rating, coupled with reports of sunscreen toxicity,3 has led to some social media influencers to advocate the avoidance of sunscreen altogether or provide ‘hacks’, where they provide recipes for their own, ‘natural’ versions.
In response to a recent Which? report, the CTPA4 underlined the strict regulatory requirements behind sunscreen development and the rigour of sunscreen development processes.
This article will outline how sunscreens work, the key requirements of a sunscreen formulation, and consider how testing for an SPF-containing product is undertaken. In addition, we’ll use our extensive experience of other industries to compare testing practices, and finally look at the incoming new In Vitro testing method – which has been 30 years in the making – set to be released in 2025!
UV Filters
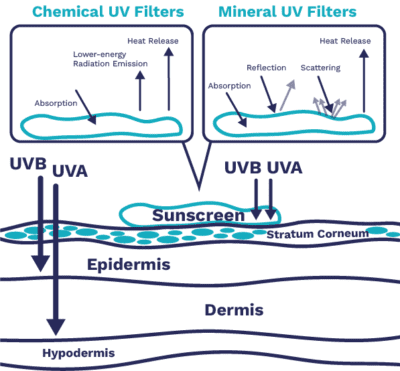
Sunscreen is a formulation to apply ingredients called UV filters to the skin. UV Filters absorb, reflect or scatter UV rays to protect the skin against UV damage. These ingredients are split into two chemical types.
Inorganic ‘Mineral/Physical’ Filters
Physical, or mineral, filters in the form of Titanium Dioxide and Zinc Oxide provide a layer on the surface of the skin, scattering wavelengths of light across the entire UV (290 – 400 nm) and visible (400 – 700 nm) spectrum.5 They can also absorb certain wavelengths of light according to the material’s bandgap, which can be tailored by the particle size.6 Mineral filters are chemically inert and photostable, with the degree of protection depending upon the amount applied; more product gives higher protection. Traditionally, mineral sunscreens were associated with opaque coverage that left white residues on the skin, so were not considered inclusive for consumers with more melanated skin. However, by reducing particle size, and creating optimum dispersions and surface coatings, today’s mineral sunscreens offer UV protection without the white marks. For example, Pretiox UVS 30 from Precheza, supplied by Cornelius, uses TiO2 nanotechnology that avoids white residues while maintaining a good UV protection profile.
Organic ‘Chemical’ Filters
Chemical compounds such as oxybenzone and butyl methoxydibenzoylmethane are common organic filters, produced for their UV protection properties. Organic filters can absorb UV energy in a range of wavelengths, so with careful selection a sunscreen can be developed to offer broad spectrum protection in both the UVA and UVB region. Organic sunscreens are usually clear and low colour. Depending upon the formulation, sunscreens containing chemical filters may be easier to apply, less sticky or greasy, and less likely to leave a white tideline.
The various regulations covering sunscreens around the globe typically have a positive list of UV filters with restrictions on max amounts that can be used. This typically results in a multiple filters being used to reach the desired SPF (UVB) and UVA protection. Physical and Organic sunscreens are often used together, particularly in high SPF products.
Sunscreen Formulation: Key Ingredients
As noted above, SPF products are no longer limited to creams used on the beach. Formulation types include creams and sprays, as well as oils, water-free sticks, and even include biphasic and powder systems. As any formulator will tell you, the final formulation is linked to the end-use: the ingredients in a UV-protective foundation will be considerably different to that of a UV protective lipstick.
In addition to providing UV protection, sunscreens are increasingly required to address safety concerns, such as extended durability or resistance to wear, or good environmental credentials. To this end, sunscreen formulations contain a range of materials such as water, emulsifiers, emollients, thickeners, film formers, rheology modifiers, and sensory amplifiers in addition to the UV filters- all of which can impact upon the SPF and stability of the final product. For example, if developing a sunscreen destined for the sports or leisure market, use of CHT-Beausil Wax 070, a 2-in-1 ingredient solution that works as an emollient and solubilizer for pigments, UV filters, and natural lipids, would be a good choice, as it allows even spreading and distribution across the skin while avoiding tackiness. In addition, the unique structure of the wax means that it forms a barrier on the skin for effective water resistance. Alternatively, CorTan DBT, a Cornelius own-brand product, is an alcohol- and oil-soluble UVB filter exhibiting exceptionally high photostability. The solubility in both alcohols and oils means that it can be easily incorporated into a range of formulations with excellent distribution throughout a batch and no risk of white residues.
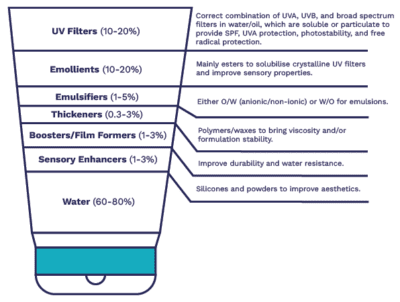
Sunscreen Formulation: Key Considerations
The main objective for a sunscreen is to protect the user from the sun’s harmful UV rays. Regardless of the sunscreen’s final state, it is imperative that any chemical or physical filters are fully solubilised, or evenly dispersed, within the formulation. As with all formulations, there are challenges to overcome, and in the case of sunscreen, an unstable formulation can have significant impact upon the end-user’s protection from solar radiation.
Ella Ceraulo, Innovation Chemist at Cornelius, shared her thoughts on the topic,
“A common misconception is that preparation of a sunscreen is simple, but this is not the case. Formulation of a sunscreen is not just the addition of UV-blockers to a moisturising lotion! As well as ensuring that the actives are evenly distributed throughout the product, the physical state of the formulation can significantly impact the final properties and performance. I shudder when I see a social media post with someone blending a zinc oxide powder into something like coconut oil to make a ‘safe’ and ‘natural’ sunscreen. This practice is dangerous and could cause serious harm.”
For example, with an emulsion-based formulation:
- Droplet size of the internal phase is impacted by the rotation speed of the mixer, the mixer’s geometry, the continuous phase viscosity, and the length of time that the emulsion is mixed. Larger droplet sizes can lead to a decrease in SPF.
- An increase in temperature either during mixing or upon storage can impact the droplet size, as well as overall emulsion stability.
- Ensuring solubility of organics and effective dispersion of actives is crucial. Any organic materials need to be fully dissolved prior to emulsion formation and any mineral materials need to be evenly distributed throughout the final product to give a uniform SPF rating.
- It is well-known that aging of emulsions can lead to flocculation, creaming, sedimentation, coalescence, or Ostwald ripening. In any lotions these outcomes impact upon shelf-life and consumer experience, but in sunscreen they can also cause a decrease in SPF efficacy.
When developing new products, the development chemists at Cornelius work closely with principal partners and customers to draw on their extensive collective experience, as well as use the Cornelius library of finished products. This speeds-up any R+D processes and product formulating, saving clients time and money.
Sunscreen Testing
All sunscreens need to comply with local legislation and regulation. Different ingredients and filters are allowed in different jurisdictions, but in all cases, a sunscreen product needs to undergo rigorous in vivo testing to fulfil regulatory requirements7. The usual route for sunscreen development includes in silico (using computer simulation), in vitro, and then in vivo testing. Each of these approaches has associated costs and benefits, and they are usually used in combination during product development.
In silico Testing
In silico testing, or computer simulation, is arguably the simplest approach and is often used to estimate the SPF of a theoretical formulation. The two most commonly used simulations are the BASF sunscreen simulator and the DSM sunscreen Optimizer. While this approach can give a rapid estimation of sun protection profile for a particular formulation and is an excellent starting point, there are some significant shortfalls. For example, the impact of other ingredients in a formulation, or the distribution of actives and pH are not considered.
In vitro Testing
In vitro testing was first developed in the 1950s and uses an artificial substrate.8 There is currently no global standard for testing and labelling UVA, which means there are several UVA test methods in use which are all In vitro9, 10. Before claiming a SPF on pack in vivo test is currently required. However, the In vitro tests are a rapid and low-cost way to collecting baseline data during product development before more expensive in vivo testing is use for product launch.
In vivo Testing
In vivo testing is considered the ‘gold standard’ in testing, and is the only method approved by regulatory bodies for determining the SPF rating of a sunscreen product. ISO (International Organization for Standardization) harmonises testing procedures, with the current EU test protocol being (ISO24444/2019). Although this technique is approved by regulators worldwide,11,12 it has some limitations.
Variation in the skin surface and skin reaction to UV radiation create variables that are difficult to control during in vivo testing. Another issue is the fact that to conduct in vivo SPF testing on human skin, it needs to be exposed to UV radiation to the point of Erythema, creating potential skin damage7, 13. Finally this type of study is far more expensive than in vitro studies, having to recruit, manage, and compensate the group of individuals who are testing the products.
What Could the Future Hold?
A new ISO test method is coming to solve the current issues with SPF testing, but let’s take a quick look at how reproducible results are achieved when testing in other product types and sectors.
Our work in the paints and coatings industries means that we are well-versed in applying samples to standardised surfaces for testing and quantifying spreading or film distribution, as well as efficacy. For example, when testing coatings the use of industry-standard Q-PANELS , such as those offered by Leneta who are the industry standard for testing charts, ensure a consistent and uniform in vitro test surface reducing the risk of variability between runs. Application can be by controlled spray or applying a known wet film thickness with an applicator like a K-bar. Vis-NIR spectroscopy could allow quantification of active distribution throughout the sample prior to in vivo testing and as a QC process during manufacture.
Recent advances in human skin-based assays, for example Skimune®, and the advent of synthetic skin (e.g. Vitro-Skin™), have led to products that are designed to mimic the surface properties of human skin including topography, pH, critical surface tension, chemical reactivity, and ionic strength. Combining expertise from Performance Coatings application with the use of synthetic skin could provide a valuable route to in vitro testing that reduces costs and improves reproducibility. There are currently no recommended procedures to substantiate durability claims, and sunscreen manufacturers are asked to develop their own robust protocols. However, the use of infrared (IR) or Raman spectroscopy in combination with 3D facial mapping could provide a quantitative method to ascertain the extent to which a product remains in place during day-to-day use.

A New Era in Sunscreen Testing
After more than 30 years of development a new in vitro ISO test is due to shake up the development of sunscreens. The new Double Plate Method (DPM) uses two plastic plates to replace human skin14. The surface of the plates have been specially adapted via moulding and sandblasting to replicate the grooves and pores of the skin’s surface. Uniform application to the plates is achieved with the use of an application robot. By using a combination of these two plates a very close correlation to in vivo results is achieved. The ISO Sun Protection Working Group (ISO/TC 217/WG 7) has recommended the new DPM and Hybrid Diffuse Reflectance Spectroscopy (HDRS) be advanced to the final draft stage. It is expected to reach final approval and be published in early 2025. Currently, this method measures UVB, so can be used to test a product’s SPF, but work is underway to be able to test UVA, which should be available in the first revision.
This new method will result in cheaper and more consistent testing. The impact of a reduced cost burden could facilitate a boom in sunscreen innovation.
Summary
The sunscreen market is rapidly expanding as consumers become increasingly aware of the dangers of UV rays and the benefits of including sun protection in their daily routine. Consumer demand for SPF in an increasingly diverse range of products has soared, with raw material suppliers, packaging suppliers, development chemists, and manufacturers continually innovating to meet this need. While this may seem overwhelming, Cornelius works with suppliers and customers to continually innovate and develop new formulations that are at the forefront of SPF technology, whether these are sun filters or blockers, or ingredients that improve product functionality, efficacy and spreadability. It goes without saying that this is an exciting area to be involved in, and an area that is set to grow.
To find out more about SPF formulating and how Cornelius can support you in creating your next SPF product with a particular target rating, please contact us at: sales.enquiries@cornelius.co.uk.
References
- Statista (2024). Sun Protection – Worldwide, Statista. Available at: https://www.statista.com/outlook/cmo/beauty-personal-care/skin-care/sun-protection/worldwide. [Accessed July 2024].
- Which? (2024). 3 sunscreens fail Which? tests, but we’ve also uncovered some Great Value options we recommend, Which?. Available at: https://www.which.co.uk/news/article/3-sunscreens-fail-which-tests-but-weve-also-found-some-great-value-options-we-recommend-aStGl7K67DKd [Accessed July 2024]
- Ruszkiewicz, A., Pinkas, A., Ferrera, B., Peres, T. V., Tsatsakis, A. and Aschner, M. (2017). Neurotoxic effect of active ingredients in sunscreen products, a contemporary review. Toxicology Reports, 4, 245–259. doi: http://dx.doi.org/10.1016/j.toxrep.2017.05.006
- CTPA (2024). You can trust your sunscreen’s SPF and UVA protection – CTPA explains why, CTPA. Available at https://www.ctpa.org.uk/news/you-can-trust–your-sunscreens-spf-and-uva-protection-ctpa-explains-why-7809. [Accessed July 2024].
- Tuchayi, S. M., Wang, Z., Yan, J., Garibyan, L., Bai, X., and Gilchrest, B. A. (2023). Sunscreens: Misconceptions and Misinformation. Journal of Investigative Dermatology, 143, 1406–1411. doi: https://doi.org/10.1016/j.jid.2023.03.1677.
- , H.-M., Lin, K.-F., Hsu, H. and Hsieh, W.-F. (2006). Size dependence of photoluminescence and resonant Raman scattering from ZnO quantum dots. Applied Physics Letters, 88, 261909. doi: https://doi.org/10.1063/1.2217925
- Zou, W., Ramanathan, R., Urban, S., Sinclair, C., King, K., Tinker, R. and Bansal, V. (2022). Sunscreen testing: A critical perspective and future roadmap, Trends in Analytical Chemistry, 157, 116724-116743. doi: https://doi.org/10.1016/j.trac.2022.116724
- Giese, A. C., Christensen, E. and Jeppson, J. (1950). Absorption spectra of some sunscreens for sunburn preparations, Journal of the American Pharmaceutical Association, 39(1), 30–36. doi: https://doi.org/10.1002/jps.3030390112
- Moyal, D., Alard, V., Bertin, C., Boyer, F., Brown, M. W., Kolbe, L., Matts, P. and Pissavini, M. (2013). The revised COLIPA in vitro UVA method. International Journal of Cosmetic Science, 35, 35–40. doi: https://doi.org/10.1111/j.1468-2494.2012.00748.x
- Wang, S. Q., Stanfield, J. W. and Osterwalder, U. (2008). In vitro assessments of UVA protection by popular sunscreens available in the United States. Journal of the American Academy of Dermatology, 59(6), 934–942. doi: https://doi.org/10.1016/j.jaad.2008.07.043.
- ISO (2019). International Standard ISO 24444 (2019) – Cosmetics – Sun protection test methods – In vivo determination of the sun protection factor (SPF), ISO.
- FDA (2021). Over-the-Counter Monograph M020: Sunscreen Drug Products for Over-the-Counter Human Use, FDA. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/omuf/OTCMonograph_M020-SunscreenDrugProductsforOTCHumanUse09242021.pdf
- Granger, C., Trullàs, C., Sokeechand, N. B., Jourdan, E., Krutmann, J., Francois-Newton, J. and Hosenally, M. (2024). Evaluating the factors influencing sun protection factors (SPF): Pooling data from multiple studies involving two reference ISO 24444:2019 sunscreen products (P2 and P8), Photodermatology, Photoimmunology and Photomedicine, 40(1), e12942. doi: https://onlinelibrary.wiley.com/doi/10.1111/phpp.12942
- How sunscreen testing is about to be transformed, Cosmetics Design, July 2024 How sunscreen testing is about to be transformed… (cosmeticsdesign-europe.com)